Business Brief
The Role of Literature Reviews in Establishing State of the Art (SOTA) for EU MDR ComplianceRevised medical device and in vitro diagnostic guidelines have challenged manufacturers looking to achieve or maintain regulatory compliance for new and existing products sold in Europe. First introduced in 2017, the MDR defines this requirement as a systematic and planned process to continuously generate, collect, analyze and assess the clinical data pertaining to a device in order to verify the safety and performance, including clinical benefits, of the device when used as intended by the manufacturer. Among the more segments of MDR are the literature review and the clinical evaluation report (CER), which are known to cause anguish for manufacturers due to the complexity and thoroughness required by notified bodies.
Literature reviews play a critical role in supporting the CER, which consists largely of clinical data collected during investigations of the product and from studies of devices deemed to be substantially equivalent to the product(s) under review. For devices currently on the market, the CER also includes references to post-market clinical follow-up (PMCF) data. Requirements for the clinical evaluation report are found in Annex XIV, Part A of Regulation (EU) 2017/745.
As part of conducting a thorough literature review and clinical evaluation report for the MDR, it is important to establish a framework that accounts for the generally accepted current knowledge, or the state of the art (SOTA). References to state of the art are found throughout Regulation (EU) 2017/745 as well as in MEDDEV 2.7/1 revision 4, an industry guidance document on best practices for MDR clinical evaluations.
Far from being a simple term that must be included or considered in literature reviews, the state-of-the-art framework plays an active role in the entire clinical evaluation process, impacting key MDR components such as risk management, demonstration of equivalence rationales, risk-benefit analysis, executive summaries, clinical investigations, literature reviews, post-market clinical follow-up, and post-market surveillance.
Defining State of the Art and Understanding Its Importance
There are nearly 40 mentions of SOTA in MEDDEV 2.7/1 revision 4, which defines state of the art as “the currently and generally agreed upon standard of care, or best practices, of the medical condition or treatment for which the device is used.”
Section 8.2 of MEDDEV 2.7/1 revision 4 describes SOTA as including applicable standards and guidance documents, data related to benchmark devices, other devices, critical components and medical alternatives or to the specific medical conditions and patient populations intended to be managed with the device. The data are typically needed in order to:
- Describe the clinical background and identify the current knowledge/state of the art in the corresponding medical field
- Identify potential clinical hazards (including hazards due to substances and technologies, manufacturing procedures and impurity profiles)
- Justify the validity of criteria used for the demonstration of equivalence (if equivalence is claimed)
- Justify the validity of surrogate endpoints (if surrogate endpoints are used)
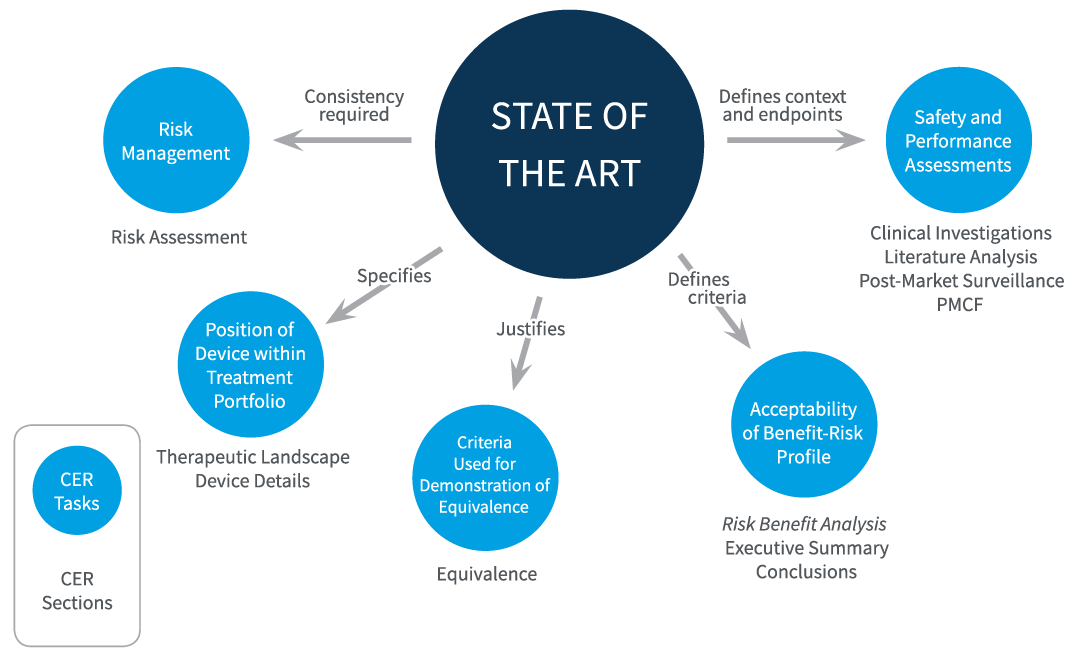
Figure 1: Role of State of the Art
Source: DistillerSR, White Paper: Best Practices and Literature Review Using DistillerSR, 2020
SOTA also includes domain knowledge such as changes to applicable standards and guidance documents, new information relating to the medical condition managed with the device and its natural course, and medical alternatives available to the target population.
Finally, it is important to note that SOTA, as it pertains to technology, does not necessarily refer to the latest, most cutting-edge tools that have been invented. Rather, the state of the art around a specific medical condition is often well-established technology that has already achieved CE mark status, having been thoroughly vetted in terms of clinical safety, performance, benefit, and risk. This stands in contrast to emerging technologies that may still be under investigation or review for CE mark approval, which, despite their relative technological advances, may still be unproven in clinical practice over time.
Having defined SOTA, it becomes clear how ubiquitous a comprehensive state-of-the-art framework should be throughout the various MDR clinical evaluation components previously mentioned. As Figure 1 indicates, a strongly defined set of currently accepted therapeutic and technological options, as well as an understanding of how these options affect the risk, benefit, safety, and performance of devices under review, can assist in providing thorough evidence of device equivalence to products already on the market. This background research justifies the results of the literature search, which require SOTA research studies, and the findings of the CER, which provides information on competitor products.
In short, SOTA serves as an underlying foundation supporting the safety, efficacy, and performance claims of the device under review. Literature reviews, meanwhile, serve as a critical data source to their timely management and submission.
Requirements and Challenges for SOTA
Despite the numerous references to state of the art in both the MDR and the MEDDEV documents, some challenges remain for manufacturers attempting to establish a SOTA framework for the product families included in their MDR submissions. Specifically, manufacturers must take deliberate efforts to avoid issues when determining which content to include in the CER to demonstrate state of the art, as well as when implementing best practices for performing a SOTA literature search to establish safety and performance and claims of device equivalence. Interpretation of what constitutes state of the art can vary within an organization.
Obtaining sufficient SOTA data via an equivalent device literature search can also pose problems, particularly for those performing literature reviews manually. Manual literature reviews are time-consuming and error-prone. They can negatively impact the clinical evaluation process by introducing potential bias, mistakes and bottlenecks that could lead to market launch delays and rejected submissions.
Here’s a step-by-step approach to establishing a SOTA framework:
- Define SOTA for Each Product Family
- Perform a Literature Search and a SOTA Search
- Framing an appropriately focused literature search question
- Performing the literature search using multiple databases
- Posing a specific SOTA question
- Conducting a SOTA search using multiple keywords and the patient, intervention, comparison, outcome (PICO) strategy
- Preparing the report that summarizes the findings
Regardless of the product being submitted for MDR certification, a CER that accounts for the SOTA framework should include a number of key sections. Specifically, background information provides the clinical context of the product family under review. This section should include summaries of the intended patient population, clinical indications and contraindications of the device(s), intended users, and specific clinical use cases for the product(s).
Within the SOTA framework, it is also important to provide treatment alternatives to the proposed medical device, which would be available for each medical condition cited in the background segment. These alternatives should be well-established, gold-standard options rather than experimental or untested products. A thorough literature search with well defined search parameters will retrieve the necessary data which can be used to establish evidence of device equivalence.
The results will lend further weight to claims of product safety and performance, particularly if the product is deemed equivalent in biological, clinical, and technical characteristics. Finally, any current standards, applicable guidelines, and evidence of the functional status of the product relative to its clinical indication can further assist in ensuring CER compliance with SOTA requirements.
These definitions will vary from one product family to the next, and sometimes from one product to another. However, the approach to thoroughly defining an appropriate SOTA framework for each product should remain consistent across the manufacturer’s portfolio.
Once manufacturers define state of the art for each product family under review, the process for arriving at a compliant literature search that can support the CER requires the following steps:
The foundation of an effective literature search is an appropriately scoped research question that returns a comprehensive yet focused and relevant list of articles. Ultimately, procuring adequate references is merely a way to ensure that the review of published data on the product family, including the SOTA, is comprehensive and not superficial.
The literature search identifies clinical data outside of the manufacturer’s domain that is needed to provide a substantial clinical evaluation of the device, justifying its candidacy for regulatory approval or compliance.
When performing the literature search, it is important to consult adequate databases in order to ensure broad capture of relevant sources. Requirements for clinical data found in the literature search are laid out in Regulation (EU) 2017/745 Annex XIV, Part A, Sections 1-4.
In contrast to a literature search, the SOTA search has a slightly different endpoint than the clinical literature review. Whereas the literature review is meant to provide evidence that justifies the safety and performance of the product for any given medical indications, the SOTA review is focused on providing evidence that the existing well-established technologies or products on the market (or the current state of the art) can be effectively compared with the device under review.
Any product outside of the specific product family under compliance review should be considered in the SOTA search rather than the literature search.
Literature search terms verifying the safety and performance of a product compare product claims (made on the product label) against clinical data (both favorable and unfavorable) from multiple sources. SOTA terms should reflect current medical practices and treatments independent of the similarity of the product(s) under review.
Once a parsed down list of relevant research abstracts has been agreed upon in the SOTA search, full-text articles must be extracted in order to fully characterize the clinical data within. This technical task can be quite burdensome for large SOTA searches, particularly when performed manually by medical writers. MDCG 2020-13, Section D indicates that full-text article extraction is a best practice to evaluate the biological, technical, and clinical characteristics of devices in use. Given the imperative nature of this task, finding practice efficiencies is thus paramount to performing a solid SOTA review.
SOTA search data quality should be assessed to ensure compliance with Annex 5, Section 3 of MEDDEV 2.7/1 revision 4 and with the requirements of proposed CER content listed in Appendix A9 of the same document.
Best Practices and Strategies For Compliant EU MDR Literature Reviews
Having complete understanding of best practices and strategies pertaining to state of the art in literature reviews is therefore imperative for MDR compliance. The purpose of literature reviews is to provide substantial evidence and clinical data to justify product claims of safety and performance compared to state-of-the-art medical treatment.
These claims can be made of new products that are being introduced into the market, enhancements or upgrades to an existing product already on the market, or competitor products deemed to be equivalent to the device under review.
Literature reviews are designed to support the CER, as outlined in Chapter VI (Article 61, Paragraph 12) of the MDR as well as in Annex II (Technical Documentation) and Annex XIV, Part A (Clinical Evaluation). CERs integrate a substantial amount of clinical data and non-clinical evidence from key findings of literature review and post-market surveillance efforts to support device safety and performance. The literature review lifecycle listed below is a consistent methodology to ensure completion of a thorough and compliant review.
Literature Review Strategies For Relevant SOTA Search
Despite the differences between the literature search and the SOTA search, there is substantial overlap between the two when it comes to the processes used to evaluate search results, research papers, references, and clinical data. The same databases, such as EMBASE and PubMed, should be used during search execution.
Adverse event databases such as the such as the Manufacturer and User Facility Device Experience (MAUDE) database (a non-EU source) should be consulted to include relevant post-market surveillance data and field performance in order to fully characterize the clinical performance of the device(s).
Initially, it will be easier to parse through research abstracts rather than full-text papers, given the volume of data that SOTA searches produce. MDCG 2020-13, Section D indicates that abstracts are a good choice for first-pass reviews. Abstracts should be triaged to determine if they are clinically relevant to the product under review (i.e., similar medical indications or use cases) or potentially equivalent devices (via biological, clinical, and technical characteristics) and if the study meets quality standards (i.e., a well-designed, conducted, and reported cohort study).
Strong rationales should be provided for articles excluded from consideration in the SOTA review to assist with traceability and further justify the methods used to establish the state-of-the-art framework.
A literature review management platform, such as DistillerSR, can help reviewers screen references by title and abstract and select relevant data. From there, they can build their inclusion/exclusion criteria, which will provide a consistent and repeatable process for determining which references to keep for the data extraction stage of the review. DistillerSR manages all the literature review data in one central repository, eliminating the need to collate individual spreadsheets and inclusion/exclusion responses for data processing and analysis. It enables reviewers to detect and remove duplicate citations preventing skew and bias caused by studies included more than once.
Annex 5, Section 3 of MEDDEV 2.7/1 revision 4 lists recommendations and methods for literature review screening. These guidelines can be used to structure adequate state-of-the-art search protocols and procedures within a manufacturer’s roadmap.
State of the art literature reviews require transparent, repeatable, and auditable processes that enable manufacturers to create and implement a standard framework.
Automated software platforms, like DistillerSR, automate the management of literature collection, screening and assessment using AI and intelligent workflows.
State of the Art, a Continuous Methodology
The SOTA review methods should be listed in the literature review protocol, as outlined in MEDDEV 2.7/1 revision 4, Annex 5, Section 3. Processes should be repeatable, in the case that new clinical data becomes available that may challenge prior claims of device equivalence, clinical safety, or performance. For example, while a product’s key claims or specifications may not change from one version to another, adverse events or substantial changes to the literature may alter the fundamental state-of-the-art framework, thus requiring a new SOTA review. The ability to archive numerous SOTA reviews is therefore an extremely useful feature of automated software programs.
When establishing a state-of-the-art framework to support a device’s clinical evaluation, the use of professional guidelines and guidance documents published by the MDCG, industry consultation groups, and the European Commission is critical.
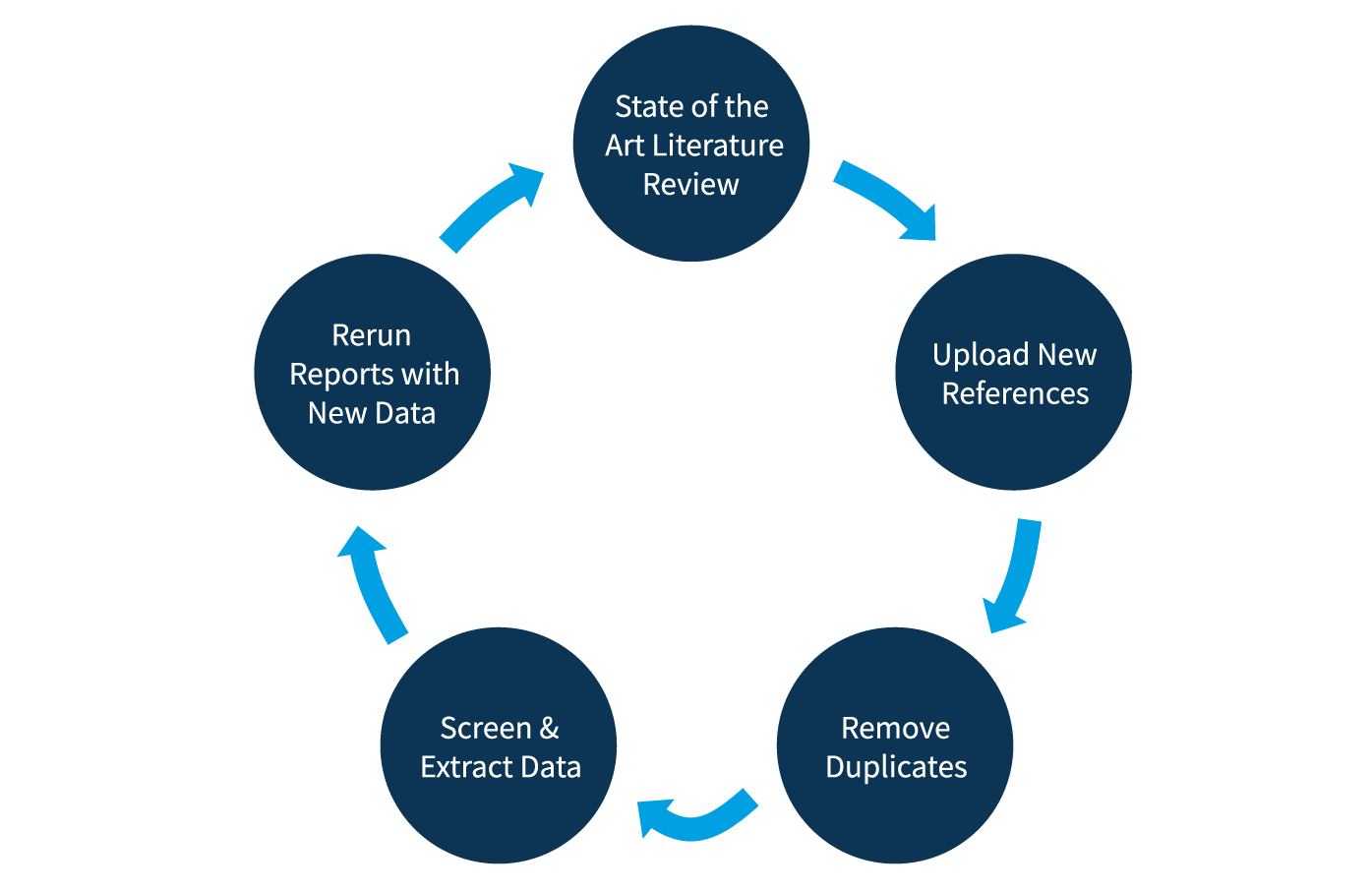
Figure 3: The Living Review Cycle
Source: DistillerSR, White Paper: Best Practices and Literature Review Using DistillerSR, 2020
Manufacturers seeking MDR compliance across a portfolio of products must also ensure the replicability of methods from one product family to another. The use of automated software designed to rapidly perform literature searches, SOTA reviews, inclusion/exclusion calculations, full-text extraction, protocol generation, and report creation can substantially improve regulatory effort efficiency, compliance rates, and cost effectiveness. These tools provide a systematic methodology that can be leveraged to ensure repeatable, consistent, and transparent processes related to the state-of-the-art framework used in the clinical evaluation of devices under MDR review.
State of the art literature reviews can be continuously maintained in the DistillerSR platform and periodically updated as new reference material becomes available.
Reviewers can automatically import newly published references, keeping literature reviews always up to date.
DistillerSR’s add-on module CuratorCR is a research knowledge center that centrally and dynamically manages an organization’s evidence-based research, allowing medical researchers to continuously collect, share, update, and reuse data across literature reviews and teams.
Ultimately, the strategies employed in establishing a state-of-the-art framework may vary from one manufacturer to the next and from one product family to another. Industry guidelines should be consulted and followed whenever possible, and the use of automated software tailored to performing SOTA literature reviews can greatly enhance the success of these complex regulatory efforts.
Download the PDF version of this business brief.
Related Resources
Blog
How DistillerSR has empowered market leaders Philips and Alcon to achieve faster and more accurate literature reviews for CER submissions.

Case Study
Dr. Bonnie H. Weiner describes the way DistillerSR reduced the time it took to complete CER literature reviews by approximately 30%.

Blog
Developing a repeatable, transparent process will lead to streamlined, audit-ready, and compliant literature reviews for your PER submissions.


