IVDR Versus IVDD

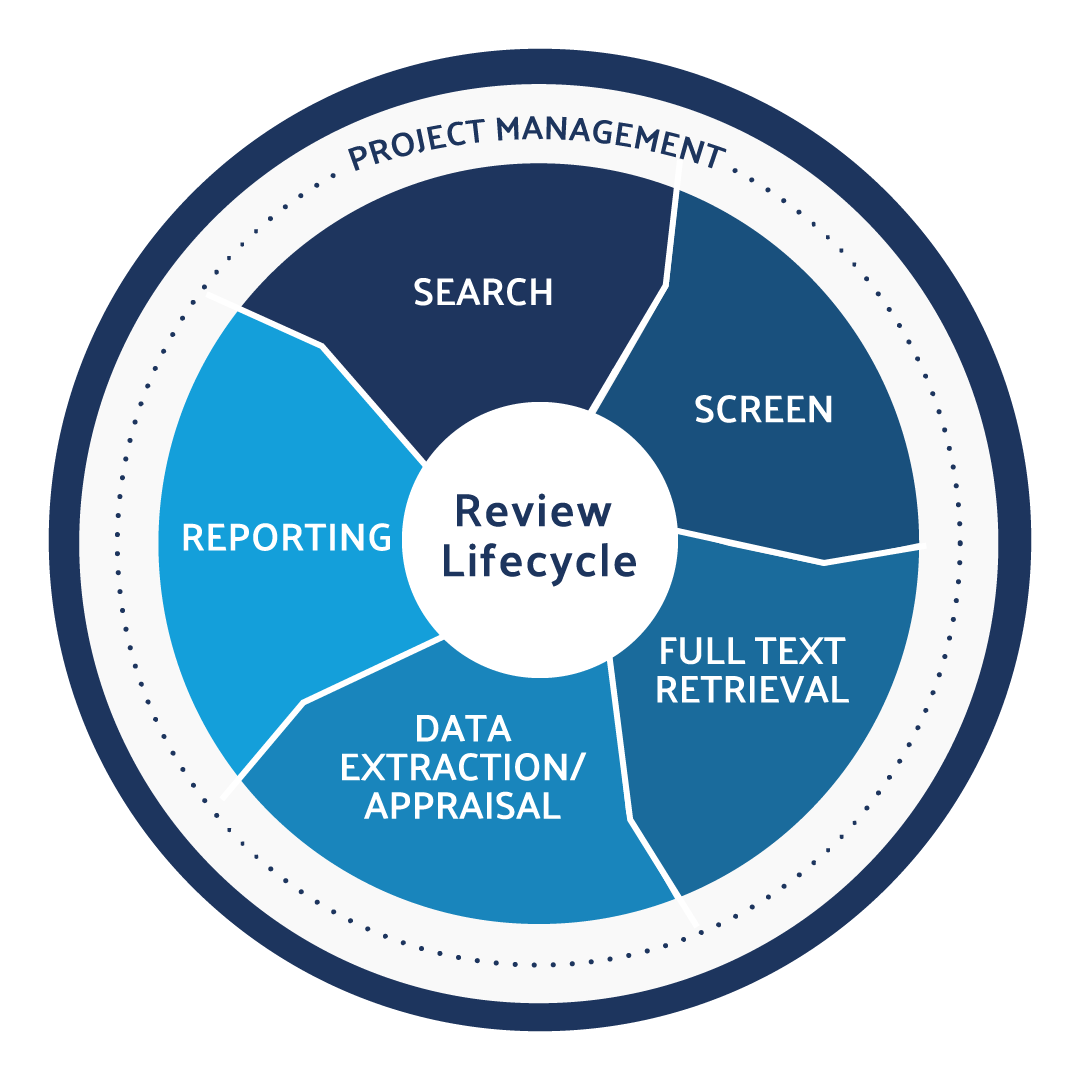
Use DistillerSR to produce CER and PER literature reviews in an efficient, audit-ready, and compliant way.
To improve clinical safety and give medical device manufacturers fair access to the market, medical regulators frequently come up with rules and regulations that must be followed by all manufacturers. These regulations are in line with the advancing medical science, production processes, and legislation put in place to ensure that patient safety is protected, while manufacturer claims against in-vitro diagnostic product (IVDs) performance are thoroughly evaluated. In-Vitro Diagnostic Regulation (IVDR) and In-Vitro Diagnostic Directive (IVDD) are among the latest regulations in the medical industry.
As a medical device manufacturer, you not only need to understand these regulations and how they affect your devices, but you also need to ensure that your devices remain compliant. The best way to prove that your medical devices have complied with these regulations is to gather all the available research data on your devices, including the available clinical studies, to generate comprehensive clinical evaluation reports.
The process of writing clinical evaluation reports can be lengthy and wearisome, especially if you are not leveraging the latest advanced tools to conduct systematic and literature reviews. It is important to start by understanding what a clinical evaluation is and the tools available to help automate the review processes for faster and more accurate completion of systematic and literature reviews. Manual systematic and literature reviews can take several years to complete and are prone to errors.
With a well-designed and reliable automated review software program, you can conduct several conclusive reviews within a year. This gives you peace of mind knowing that your IVDs have complied with the necessary regulations. Apart from incorporating the right tools and programs into your systematic and literature reviews, you also need to be aware of the latest rules and regulations in the industry. For instance, you should be familiar with key differences between the IVDR and IVDD regulations.
What Is IVDR?
The IVDR is the In-Vitro Diagnostic Regulation relating to in-vitro diagnostic medical devices in the European Union (EU) market. The IVDR, which became operational on 26 May 2017, with a five-year transition period, was meant to replace the EU Directive (98/79/EC) that had been regulating in-vitro diagnostic medical devices (IVDD) since 1993.
The IVDD lacked the necessary provisions to regulate novel devices and cover the progress that was taking place in the medical industry. Therefore, the EU saw the need to come up with more comprehensive regulations to replace the directive, hence the creation of IVDR. So, how does the IVDR regulate IVDs while taking into account the ongoing scientific changes?
First, a five-year transition period ensured that medical device manufacturers had enough time to adopt the innovations and pathways to promote their new products. The suppliers also had sufficient time to comply with the new requirements. The IVDR is expected to improve the safety, consistency, and quality of IVDs. This regulation also advises manufacturers on how to classify their IVDs based on the risk. Therefore, you need to find a comprehensive IVDR classification guide to understand the different classes and devices in each one.
The IVDR offers new definitions of IVDs and improves their classification, surveillance, and regulation. With IVDR, algorithms satisfying these classifications are structured as IVDs. However, it is still unclear how the algorithms will be regulated under the IVDR. In short, IVDR has raised the standards and increased the regulatory scope of IVDs.
Learn More About DistillerSR
(Article continues below)
What Is IVDD?
The IVDD is an EU directive created in 1993 to regulate IVDs. Every IVD manufacturer must ensure that their medical diagnostic devices meet all the requirements outlined in the directive, including quality, designs, packaging, production, and labeling requirements. Although IVDD has the same impact on manufacturers and IVDs as IVDR, as they share the same basic regulatory process, IVDD does not cover as many areas as IVDR.
The IVDD, which is being replaced by the IVDR, used a list-based approach to classifying IVDs based on risk. This approach determined the procedure for evaluating compliance and the level of review needed from a Notified Body (NB). The IVDR, on the other hand, uses internationally recognized regulations to assign every device to each of the risk classes.
Please note that some IVDs issued with valid certificates under the IVDD can continue to be sold up to May 26th, 2024, albeit under specific conditions. They can also remain available in the market until May 26th, 2025. Also, manufacturers of IVDs can make their devices available on the market under the new regulation even before its Date of Application (DoA), as long as they comply with it.
Lastly, note that different articles of the IVDR have specific dates of application. For instance, Article 100 on EU reference labs for IVDs was introduced on May 25th, 2020. Given the differences between IVDD and the newer IVDR regulations, it is imperative to review and understand every article of the regulation, to ensure that all your IVDs remain compliant.