What’s New in DistillerSR’s Latest Release
Systematic reviews take time to reach completion as they require comprehensive synthesis of evidence and follow a protocol. However recent global events have compelled the research and healthcare industries to provide faster results, while dealing with a consistently growing volume of references. Consequently, research professionals are looking for greater review efficiencies and transparency in collecting and reporting on validated data. In the latest DistillerSR update, we have added new capabilities that enable you to manage evidence in an easy manner, and automatically generate comprehensive PRISMA 2020 flow diagrams for your projects’ reports. The result: improved quality of reviews while saving you significant time, effort and cost. Here is what’s new…
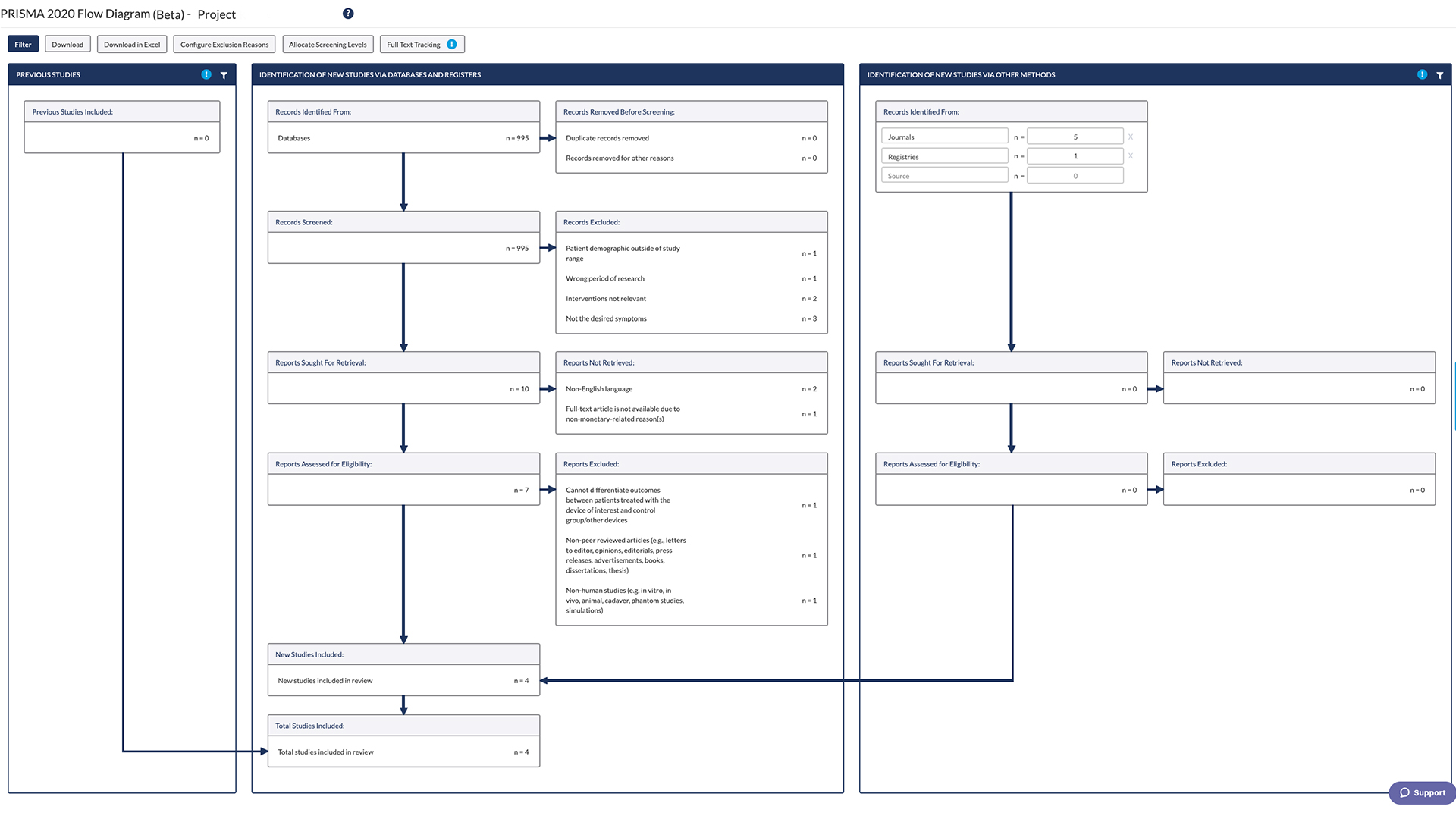
PRISMA 2020 Flow Diagram- Transparent, Accurate and Comprehensive
DistillerSR has been providing users with the ability to automatically generate their PRISMA flow diagram and its calculated numbers based on the decisions made and captured when using DistillerSR to conduct their screening. So when PRISMA 2020 guidelines were published, DistillerSR upgraded its flow diagram to support and facilitate this version and its new components.
We understand that reviewers’ requirements for creating a report are complex and diverse. Built and improved with customer input, DistillerSR brings the industry’s most comprehensive PRISMA 2020 flow diagram that is:
- Highly Configurable
- Accurate
- Editable, yet secure
- Clear, clean and easy to read
DistillerSR captures the essence of the PRISMA 2020 report guidelines and makes easy work of it, vastly reducing the time and effort it takes to create, calculate numbers and update the flow chart manually. It enables you to generate a detailed and transparent report that helps readers understand what was done and why, and supports clinicians and decision- makers implement results in practice.
CuratorCR- Always Accessible And Reusable Data
Data collection and processing is a huge investment of resources. By building on the findings of previous research, you can dramatically accelerate your own work, making you more efficient, productive and cost effective.
CuratorCR is a research knowledge center that dynamically manages an organization’s evidence-based data by consistently collecting, sharing, updating and reusing them– a single source of truth for reviewers.
Integrated seamlessly with DistillerSR, CuratorCR actively promotes reuse of previously collected data to reduce research time and improve reviewer productivity by proactively identifying available data and making suggestions (Answer Suggestions) to reviewers in real-time.
Organizations can segment their CuratorCR instance into repositories to provide or limit access to trusted review data as opposed to the current status quo of ad-hoc, unorganized and static storage of data.
Do you want to see the updates in action? Book a free demo to learn more about DistillerSR today!