Business Brief
Smart Automation of SLRs for GVDs: Faster Market Access for New DrugsHealth technology agencies (HTAs) and payers demand detailed evidence for the conclusions health technology companies make about new products, requiring comprehensive justification for the assumptions, comparator(s), and criteria used to assess its potential value. Pharmaceutical, medical device, and diagnostics developers consolidate the evidence for the value of their innovations into a global value dossier (GVD), which informs submissions to HTA and payer agencies.
Systematic literature reviews (SLRs) are key sources of information for GVDs. These reviews generate large quantities of data that must be vetted, captured, categorized, analyzed, and updated regularly, in what can be a time-consuming, labor-intensive, and expensive ongoing process. This business brief reviews how automating aspects of the SLR can improve its accuracy and efficiency while supporting the development of a targeted, fit-for-purpose dossier.
The central tension in preparing a GVD is between inclusivity and relevance. Too much information makes the document unwieldy. Too little information renders it less valuable. A subset of this issue is the tension between local applicability and international reach – the need to include all information for relevant local affiliates as well as all global information while producing a usable document.
The Global Value Dossier: Background
A GVD states the value proposition for a life sciences product and supports that proposition with evidence about the global burden of the condition addressed by the product, current treatment, and unmet needs, as well as the evidence of the product’s clinical, economic, and humanistic (e.g., patient-reported outcomes [PROs], quality of life) value compared with the standard of care or other appropriate comparator(s).
The GVD is created by the product developer’s global headquarters and typically focuses on global information but also includes country-specific data based on input from local affiliates. It forms the basis for market access teams and others in life sciences firms to communicate the value proposition to local, regional, or national payers; HTA agencies; and other authorities that control or affect market access and reimbursement. The dossier therefore provides crucial support for patient access to new therapeutics, diagnostics, and devices.
Early GVD development may promote communication among internal teams so that clinical trials include research questions relevant to HEOR and market access needs. Otherwise, clinical trials may conclude without gathering data needed for the GVD, such as PROs, cost-benefit analysis, or quality-adjusted life year.
Results of a survey of global pharma employees responsible for health economics and outcomes research, market access, or reimbursement illustrate this tension.
Fewer than one-third of respondents who had a GVD available when they last developed a local HTA submission believed that the GVD offered value, with only 31% (7/23) considering it “extremely” or “very” valuable. Factors cited as limiting a GVD’s value for preparing a local HTA submission were lack of relevance to the country/region (57%), lack of comprehensive content (39%), language barriers (35%), cumbersome format (30%), and outdated content (22%).1
These responses suggest a need for comprehensive inclusion of the relevant content in a usable form, with frequent updating. All content should support the value proposition without extraneous material. The GVD must feature local treatment guidelines and evidence related to the relevant geographic regions yet be structured for easy navigation.
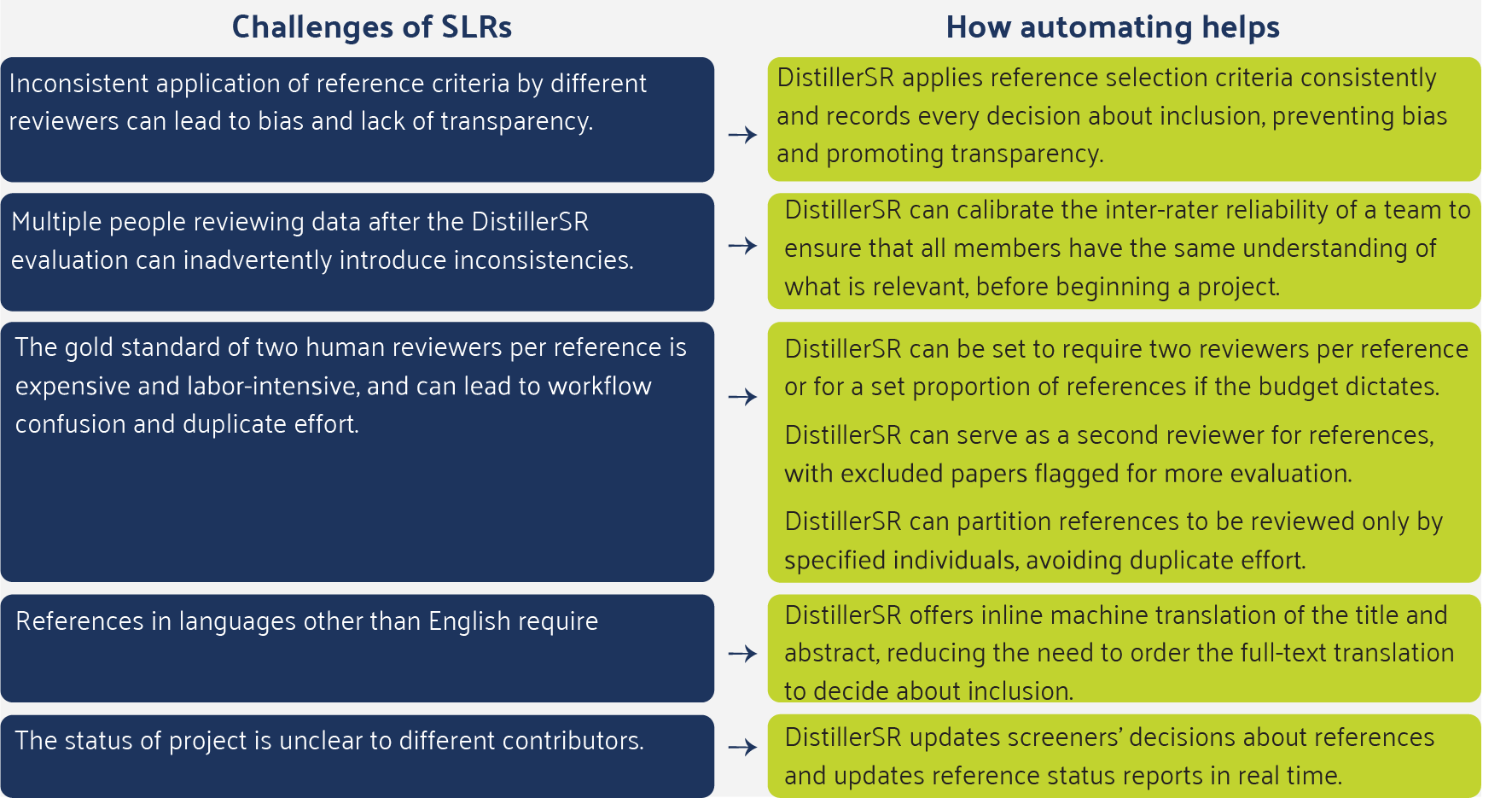
SLRs are commonly used to gather evidence for the GVD sections covering disease burden, current treatment, and unmet needs, as well as the clinical, economic, and patient impact sections. The quality, comprehensiveness, and relevance of the evidence in the SLR is the foundation of the subsequent analyses and conclusions. Characteristics of a quality literature review include:
- Comprehensive inclusion of all relevant references.
- Transparency in reference selection criteria and application of criteria.
- Consistent application of reference selection criteria, without bias.
- Dual screening of each reference.
Preparing a global value dossier can involve reviewing hundreds or thousands of references – screening, reviewing, and extracting data and categorizing each document.
The process of human screeners and reviewers performing these tasks using spreadsheet software without automation inevitably leads to errors, such as inconsistent application of selection criteria and keyword choices, inconsistent categorization of references, and copy-paste errors.
The inefficiency of humans working remotely across time zones, rather than collaborating in real time, can also lead to accidental duplication of work.
A popular literature review platform used by 60% of the top pharmaceutical companies and many contract research organizations is DistillerSR. DistillerSR’s cloud-based platform automates the literature review process through AI-powered automation and intelligent workflows to simplify and accelerate the review process, allowing researchers to work faster and more accurately than traditional methods. Below are some of the challenges with developing SLRs that automation addresses.
Information specific to local affiliates can be included in appendices or in links to online data repositories. This structure avoids lengthening the dossier to an unusable size while providing local offices what they need. Local affiliates’ access to a central data repository such as DistillerSR enables selection of the appropriate local data by including links rather than all data in the document.
Importance of an evidenced-based GVD to market access
Erring on the side of brevity in a GVD can be risky, as HTA and payer agencies demand comprehensive information not always found in GVDs. Omitting that information from the life sciences firm’s HTA or payer agency submission can reduce the chance of reimbursement.
A consulting firm cites these five common global HTA template requirements that GVDs often address inadequately:2
1. Inadequate justification of the choice of comparator, patient relevance and endpoint validity, generalizability of the data, and economic model inputs.
2. Incomplete trial and analysis information.
3. Insufficient information on patient benefit.
Consider linking data about the burden of the disease on patients to findings demonstrating that the product improves patient-reported outcomes and quality of life parameters related to that disease burden.
4. Economic impact.
Even HTA agencies that do not assess cost effectiveness may appreciate information about the cost, materials, and training required to use the new product compared with the current standard of care.
5. Evidence summaries.
These can appear along with the detailed information mentioned above, for agencies and officials who prefer that format.
- Sarnes E, Shields K, Kaufman S, Meyer KL. Global value dossiers (GVDS): How to ensure the value isn’t lost in translation. Poster presented at ISPOR Europe 2016; October 29 to November 2, 2016; Vienna, Austria.
- Chadwick, C. Do your global value dossiers contain these 5 common omissions? PRMA Consulting. Available at: https://www.prmaconsulting.com/blog/creating-a-high-quality-global-value-dossier-support-your-affiliates-by-avoiding-common-omissions-and-focusing-on-local-hta-requirements/.
Download the PDF version of this solution brief.
Related Resources

Case Study
With as many as 40,000 references per project, Medlior, a HEOR consultancy, used DistillerSR to more accurately and efficiently manage their literature reviews.

Business Brief
Information Overload Drives New Approaches to Managing HEOR Literature Reviews.

Webinar
How Information Overload is Driving New Approaches for HEOR Systematic Literature Reviews.


